Copper's effects on the intergranular corrosion of aluminium alloy 6005
The increasing use of 6xxx series aluminium alloys in automotive applications is due to its high strength-to-weight ratio, good extrudability and good overall resistance to corrosion. Nevertheless, these alloys may become susceptible to intergranular corrosion due to unfavorable alloying and thermomechanical treatment. Let’s look at this issue.
Copper segregates as a Cu-rich nanolayer, together with the formation of a solute-depleted zone, along the grain boundaries during thermomechanical processing of the aluminium alloy. While the α-phase particles corrode in acidified chloride test solution, additional copper becomes enriched as nanoscale particles and layer segments by dealloying on the external surface and fissure walls formed by intergranular corrosion (IGC).
While the grain-boundary layer functions as the internal cathode, enriched copper acts as effective external cathodes in IGC propagation.
Effects of copper in trace amounts
I’ve been part of a research team that has investigated the significance of small copper (Cu) content on the formation of local cathodes and their function in determining the IGC propagation mechanism of alloy AA6005-T5 in acidified chloride solution. This is 6005 aluminium in the T5 temper.
Cu content in trace amounts – as low as 0.14 wt% – can cause susceptibility, depending on the temper and type and amount of other alloying elements. This susceptibility has been related to the segregation of a copper-rich layer with the thickness of a fraction of a monolayer along the grain boundaries and a solute-depleted zone adjacent to it, which gives rise to micro-galvanic coupling as the local driving force for IGC.
In related work performed earlier, Cu was shown to become enriched at the surface of the corroding α-phase particles due to dealloying of the more active aluminium, iron and manganese components in acidified chloride solution. However, the remaining Cu in the form of a debris of Cu flakes did not continue to function as the external cathodes because of poor contact with the underlying Al matrix.
Different surface pre-treatment, such as mechanical polishing, argon sputtering and alkaline etching, does not lead to significantly different initiation of IGC.
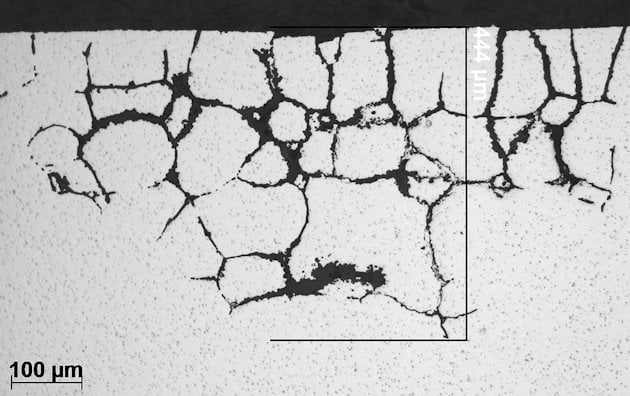 General cross section image of intergranular corrosion in 6xxx alloy
General cross section image of intergranular corrosion in 6xxx alloy
Cathodic activity increases
The cathodic activity during IGC propagation on AA6005-T5 with small copper content, increases with the time of immersion in acidified chloride solution. This is caused by enrichment of copper in the propagating IGC filaments, and on the outer surface, as a result of dealloying.
Initially, the α-phase and the Cu-rich nanolayer along the grain boundaries are the external and internal cathodes, respectively, in driving intergranular corrosion. The α-phase corrodes, and exposure of a fresh α-phase particle is delayed because of the distance between the particles in the alloy. Meanwhile, Cu enriches continuously by dealloying at the corroding sites and becomes the dominating cathode both externally and internally.
The difference in the etching morphology between the external surface and the IGC filaments suggests indirectly that the pH is lower, and the chloride concentration higher in the filaments than those in the bulk solution. This can occur only by participation of the external cathodes in the propagation of IGC. Thus, the principle of separation of the anodic and cathodic sites in localized corrosion is satisfied.